Data from a year-long Phase I clinical trial suggest that a donor cell therapy for people who are receiving liver transplants can safely calm the recipient’s T cells and may reduce the risk of their immune system rejecting the transplanted organ. The trial found that patients who received donor-derived regulatory dendritic cells (DCreg) infusions exhibited a drop in anti-transplant immune responses that was similar to that of individuals who received standard immunosuppressive (IS) drugs.
The early-stage clinical trial results, reported by University of Pittsburgh School of Medicine scientists, point to a path that may spare organ transplant recipients from the serious side effects of long-term immunosuppressant use—which can include cancer, diabetes, kidney failure, and susceptibility to infections—allowing them to be weaned off immunosuppressants without rejecting the transplanted organ.
“These trial results are very encouraging,” said Angus W. Thomson, PhD, DSc, distinguished professor of immunology and surgery at Pitt and member of the Thomas E. Starzl Transplantation Institute. “Right now, we’re seeing preliminary evidence that this intervention is modifying the recipient’s immune response in such a way that we may be able to safely reduce—or even withdraw—immunosuppression. It would be a significant service to the transplantation community if patients are no longer dependent indefinitely on immunosuppressants.” Senior author Thomson and colleagues outlined the study details and results in Science Translational Medicine, in a paper titled “Donor-derived regulatory dendritic cell infusion modulates effector CD8+ T cell and NK cell responses after liver transplantation.”
The “remarkable success” of organ transplantation as the treatment of choice for end-stage disease is related to the development of safe and effective antirejection agents, the authors noted. “However, immunosuppressants in current use suppress host responses nonspecifically and, with rare exceptions, must be taken indefinitely to ensure graft survival.” Individuals taking antirejection treatment over the long term may be susceptible to serious complications of infection, cancer and potentially serious off-target side effects, including cardiovascular issues. “Thus, despite excellent (>90%) kidney or liver allograft survival at one year, long-term outcomes remain poor, with an average of only 60% graft survival at 10 years,” the team noted. Enabling better log-term graft and patient survival will be dependent on the development of strategies that induce donor-specific immune tolerance, and will allow donor recipients to reduce or stop immunosuppressive therapy within a few years.
The ability of the liver to regenerate means that people can donate a portion of their liver to someone else in need. Both the part of the liver left in the donor and the part given to the recipient will regrow to full-sized livers. This is called a living donor liver transplant, or LDLT.
For the reported Phase I trial, carried out by Thomson in collaboration with Abhinav Humar, MD, clinical director of the Starzl Transplantation Institute and chief of the Division of Transplantation at UPMC, and colleagues, 15 patients who were scheduled to receive LDLT also received the donor DCreg infusion. These “STUDY” patients were then compared with another 40 standard of care (SOC) patients receiving LDLT who were not given the donor immune cell infusion.
For the trial procedure, several weeks before surgery, the research team took blood from the donors for the trial patients and separated out the monocyte white blood cells. They then induced the monocytes to make regulatory dendritic cells, a type of immune cell that helps the rest of the immune system distinguish foreign invaders that must be eliminated from parts of the body that should be left alone. “Regulatory dendritic cells (DCreg) are innate immune cells that down-regulate alloimmune responses in preclinical models” the authors explained. “… there is a strong rationale for assessing the ability of DCregs to attenuate host immune reactivity that may permit safe minimization or early withdrawal of immunosuppressive (IS) therapy in clinical organ transplantation.”
A week before the liver transplant procedure the newly made DCregs were infused into the liver recipient patients. The liver transplant then proceeded as normal and the patients were given immunosuppressant medications just like they would have if they had not received the DCregs.
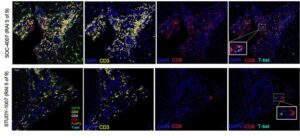
The researchers also checked for differences in immunologic activity between the two patient groups. A year after the transplantation, they found that the patients who received the DCreg infusion exhibited a reduction in other immune cells that would signal a negative reaction to the transplanted liver. In animal studies this reduction has enabled researchers to successfully wean animals off immunosuppressants. “The downmodulation of effector CD8+ T cell and NK cell responses observed may be conducive to reduced dependence on IS drug therapy and induction of operational transplant tolerance,” they wrote.
Interestingly, the transplanted DCregs persisted in the recipient patients for only a few days. But during that time, they were able to produce exosomes that allow cells to communicate by transferring messages from one cell to another, influencing a variety of cellular behaviors. “We believe that these donor-derived exosomes are preemptively conditioning the prospective LDLT recipient to see donor cells as safe,” said Thomson. “A year post-transplant, clinicians will then determine which patients can start tapering off immunosuppressants. Then time will tell if our approach works.” The authors further noted, “Analyses of additional DCreg-infused patients are now needed to extend mechanistic understanding in relation to clinical outcomes that may underpin further evaluation and development of this innovative approach to cell therapy in organ transplantation.”
Thomson and the team continue to follow the trial participants and plan to report more results in about a year.