Researchers from University of California San Diego School of Medicine have used single-cell RNA sequencing (scRNA-seq) to identify a pattern of gene expression that can be used to predict whether or not neurons will regenerate after an injury. Tests in mice showed that this “Regeneration Classifier” was consistently reliable in predicting the regeneration potential of neurons across the nervous system and at different developmental stages. Conditional gene deletion then validated a role for NFE2L2 (or NRF2), a master regulator of antioxidant response, in corticospinal tract regeneration.
“Single-cell sequencing technology is helping us look at the biology of neurons in much more detail than has ever been possible, and this study really demonstrates that capability,” said senior author Binhai Zheng, PhD, professor in the Department of Neurosciences at UC San Diego School of Medicine. “What we’ve discovered here could be just the beginning of a new generation of sophisticated biomarkers based on single-cell data.” Zheng and colleagues reported on their findings in Neuron, in a paper titled “Deep scRNA sequencing reveals a broadly applicable Regeneration Classifier and implicates antioxidant response in corticospinal axon regeneration.” In their paper the team concluded, “Our data demonstrate a universal transcriptomic signature underlying the regenerative potential of vastly different neuronalpopulations and illustrate that deep sequencing of only hundreds of phenotypically identified neurons has the power to advance regenerative biology.”
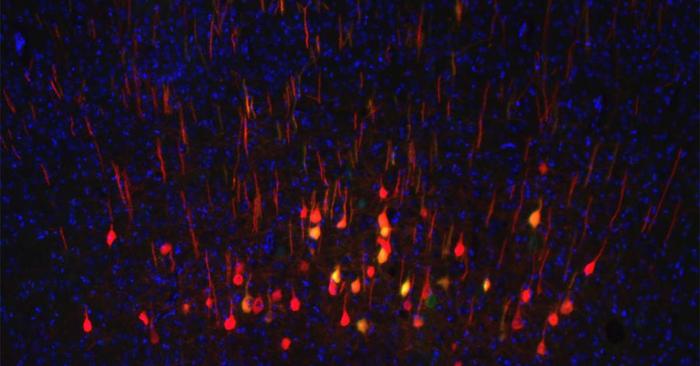
Neurons are among the slowest cells to regenerate after an injury. While scientists have made progress in understanding neuronal regeneration, it remains unknown why some neurons regenerate and others do not.
For their study the researchers focused on neurons of the corticospinal tract, which is a critical part of the central nervous system that helps control movement. After injury, these neurons are among the least likely to regenerate axons—the long, thin structures that neurons use to communicate with one another. This is why injuries to the brain and spinal cord are so devastating. As the authors noted, “Despite substantial progress in understanding the biology of axon regeneration in the CNS, our ability to promote regeneration of the clinically important corticospinal tract (CST) after spinal cord injury remains limited.”
First author Hugo Kim, PhD, a postdoctoral fellow in the Zheng lab, added, “If you get an injury in your arm or your leg, those nerves can regenerate and it’s often possible to make a full functional recovery, but this isn’t the case for the central nervous system. It’s extremely difficult to recover from most brain and spinal cord injuries because those cells have very limited regenerative capacity. Once they’re gone, they’re gone.”
To carry out their investigations the researchers used Patch-based single-cell RNA sequencing to analyze gene expression in neurons from mice with spinal cord injuries. They encouraged these neurons to regenerate using established molecular techniques, but ultimately, this only worked for a portion of the cells. This experimental setup allowed the researchers to compare sequencing data from regenerating and non-regenerating neurons.
Further, by focusing on a relatively small number of cells—just over 300—the researchers were able to look extremely closely at each individual cell. “Just like how every person is different, every cell has its own unique biology,” said Zheng. “Exploring minute differences between cells can tell us a lot about how those cells work.”
Using a computer algorithm to analyze their sequencing data the researchers identified a unique pattern of gene expression that can predict whether or not an individual neuron will ultimately regenerate after an injury. The pattern also included some genes that had never been previously implicated in neuronal regeneration. “It’s like a molecular fingerprint for regenerating neurons,” commented Zheng.
![Hugo Kim, PhD (left) designed and executed the single-cell RNA sequencing experiments under the supervision of Binhai Zheng, PhD (right). [UC San Diego Health Sciences]](jpg/low-res-4-300x157.jpg)
To validate their findings the researchers tested this molecular fingerprint, which they named the Regeneration Classifier, on 26 published single-cell RNA sequencing datasets. These datasets included neurons from various parts of the nervous system and at different developmental stages.
The team found that with few exceptions, the Regeneration Classifier successfully predicted the regeneration potential of individual neurons and was able to reproduce known trends from previous research, such as a sharp decrease in neuronal regeneration just after birth. “We found that our Regeneration Classifier can be applied in an unbiased manner to characterize any published scRNA-seq dataset,” the team commented. “This generated a pattern of regeneration classification for various neuronal populations that remarkably reflects prior knowledge on their regenerative potential based on the neuronal type and developmental stage.”
Zheng added, “Validating the results against many sets of data from completely different lines of research tells us that we’ve uncovered something fundamental about the underlying biology of neuronal regeneration,” said Zheng. “We need to do more work to refine our approach, but I think we’ve come across a pattern that could be universal to all regenerating neurons.” In their paper the investigators stated, “It has been extensively shown in the literature that neurons undergo a developmental stage-dependent decline in regenerative abilities. However, our data provide, for the first time to our knowledge, a transcriptomic basis for this phenomenon across vastly different neuronal types.”
A closer evaluation of differentially expressed genes, and gene network analyses, highlighted a gene called NFE2L2 (nuclear-factor-erythroid-derived-2-like 2) also known as NRF2 (nuclear-factor erythroid-2-related factor 2), as a potential regulator of differentially expressed genes in regenerating, compared with non-regenerating CST neurons. NFE2L2 encodes a transcription factor that activates antioxidant genes that are under oxidative stress in response to injury and inflammation. Tests in engineered mice indicated that NFE2L2 acted as a positive regulator of CST regeneration. The team said the findings “… validate our Patch-seq approach in discovering new regeneration regulators.”
Another top candidate PPARGC1A (or PGC-1α), encodes a master regulator of mitochondrial biogenesis, but the role of PGC-1α in CST regeneration has yet to be validated in vivo, the authors noted. Nevertheless, they stated, “… our observation that these two genes (NFE2L2 and PPARGC1A) sit at the top of the regulatory network in regenerating neurons highlights the importance of both antioxidative response and mitochondrial biogenesis.”
While the results in mice are promising, the researchers caution that at present, the Regeneration Classifier is a tool to help neuroscience researchers in the lab rather than a diagnostic test for patients in the clinic. “For preclinical studies, a Regeneration Classifier may serve as a biomarker to predict the likelihood of success for candidate regenerative therapies. In this regard, our approach will likely have broad applicability in studying many other neurological conditions,” the scientists stated.
“There are still a lot of barriers to using single-cell sequencing in clinical contexts, such as high cost, difficulty analyzing large amounts of data and, most importantly, accessibility to tissues of interest,” said Zheng. “For now, we’re interested in exploring how we can use the Regeneration Classifier in preclinical contexts to predict the effectiveness of new regenerative therapies and help move those treatments closer to clinical trials.”