By Sally Temple
The Nobel Prize–winning discovery that mature, specialized cells can be reprogrammed to lend them pluripotency (that is, the ability to develop into all tissues of the body) unleashed vast potential in the fields of disease modeling, drug screening, regenerative medicine, and wound healing. The reprogrammed cells are known as induced pluripotent stem cells (iPSCs). In culture, pluripotency must be maintained when iPSCs undergo proliferation in advance of differentiation. Production of large, homogeneous quantities of iPSCs and their differentiation into specific cell products requires precise control of culture conditions, including the concentration, sequencing, and timing of a variety of growth factors and cytokines.
Ensuring a steady concentration of the growth factors and cytokines needed to drive proliferation and preferential differentiation can be time consuming and labor intensive. These proteins are highly labile, with each having a different half-life in cell culture media. When the proteins are added to a culture vessel, their concentrations peak and then rapidly decline. At the same time, the ratios of factors change, altering cell signaling patterns.
When the proteins are replenished by media exchanges, the same pattern repeats. Consider the impact of fibroblast growth factor 2 (FGF2) on iPSCs, which has a half-life of approximately 4 hours in culture medium. At a relatively high concentration, FGF2 provides a pluripotency signal to iPSCs; at a relatively low concentration, it signals differentiation. Peaks and troughs of FGF2 concentration resulting from medium exchanges can lead to the iPSC population getting “mixed signals” and becoming increasingly heterogenous, the opposite of what is needed.
Control culture conditions
FGF2 DISC™ devices consist of biodegradable StemBeads® loaded with recombinant human FGF2 embedded in an inert nonbiodegradable, biocompatible hydrogel. Placed directly into the cell culture vessel, DISC devices enable precise control of the concentration of FGF2 throughout the duration of an experiment, creating a more physiologic environment (Figure 1). StemBeads contained within the DISC device release growth factor at a controlled rate, minimizing variations in concentration and reducing the resulting stress on cells. DISC devices do not degrade and can be easily added or removed from culture vessels.

contain different growth factors or cytokines at different concentrations. (The schematic was created using BioRender.)
FGF2 DISC devices are particularly useful in stem cell and iPSC cultures where they reduce spontaneous differentiation, enhance pluripotency, and guide preferential differentiation. When culturing iPSCs, one DISC device per 2 mL of medium will deliver a steady level of 10 ng/mL FGF2. Medium is replaced every two to three days using a low-powered vacuum or pipette, leaving the original DISC device in the well. After about seven days, cells are passaged into a new culture dish and a new DISC device is added, while the old one is discarded.
FGF2 DISC devices are compatible with a variety of stem cell media and are stable for six months when stored at either 4°C or −20°C.
Enhance pluripotency
High-quality, homogenous cultures of iPSCs are essential for efficient differentiation into endoderm-, mesoderm-, and ectoderm-derived cell types. Use of a DISC device to deliver a steady state of FGF2 to cultures of iPSCs results in the desired tight cell colonies expressing pluripotency markers OCT4, SOX2, and NANOG (Figure 2A) and having normal karyotype (Figure 2B). Flow analysis shows enhanced levels of pluripotency markers (SSEA-4) and a reduction in the level of the undesired marker SSEA-1, a cell surface protein involved in cell differentiation, as compared to manual, daily replenishment (Figures 2C & 2D).
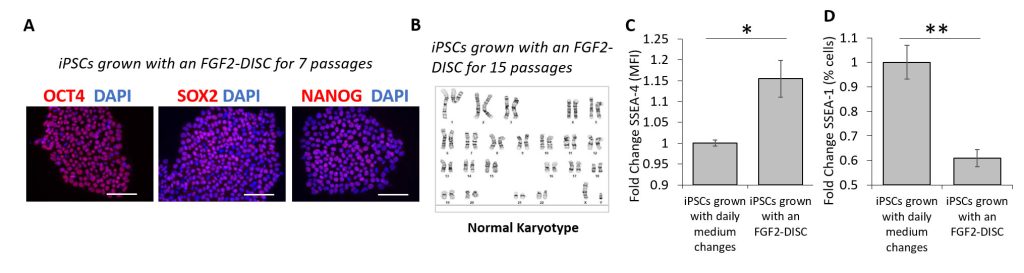
These results demonstrate the ability of DISC devices containing StemBeads loaded with high-quality FGF2 to enhance the pluripotency of iPSCs and reduce spontaneous differentiation. Note that this enhancement is observed when using FGF2 DISC devices even with just one or two medium changes per week.
Improve quality following cryopreservation
The ability to cryopreserve iPSCs without compromising their normal biochemical and physiologic functions is essential to support their various applications. Although there are robust cryopreservation protocols available for iPSCs, the cells can sometimes undergo unwanted, spontaneous differentiation shortly after thawing and passaging. When the cells are spontaneously differentiating, they may express SOX17, which is an endoderm marker; if the desire is to produce mesoderm or ectoderm, the presence of SOX17-positive cells will reduce efficiency.
Additional experiments were run to compare iPSCs that had been cryopreserved, thawed, cultured for seven days, passaged, and grown for two additional days in mTeSR™ medium either with (Figure 3A) or without (Figure 3B) an FGF2 DISC device. The culture without an FGF2 DISC device was fed every day with mTeSR medium to replenish FGF2.
In this daily fed culture, there was a significant amount of spontaneous differentiation as evidenced by the flat cells with various morphologies around the edges of the colonies (Figure 3B). A culture with spontaneous differentiation of iPSCs must be cleaned prior to differentiation, a time-consuming, laborious process in which the undesirable differentiated colonies are scraped away or the undifferentiated colonies are lifted out. In contrast, the colonies in the culture containing the FGF2 DISC device and receiving just one to two feeds per week are tighter with much less spontaneous differentiation, indicating a more pluripotent state (Figure 3A).

the cultures with an uncontrolled FGF2 concentration.
Reduce costly media consumption
In addition to enhancing the pluripotency of iPSCs, use of DISC devices reduces costly media consumption and the labor necessary to perform frequent media changes. By incorporating DISC devices into culture protocols, the number of media changes can be reduced, leading to significant cost savings, especially for large-scale production. Fewer media changes also minimize the risk of contamination created by handling and opening cell culture vessels.
iPSCs have proven to be a powerful tool for both research applications and development of therapeutics. Growth of these cells in culture can be challenging, requiring frequent feeding and precise, sustained concentrations of growth factors to maintain pluripotency and minimize spontaneous differentiation. DISC devices are a novel solution for maintaining consistent concentrations of critical growth factors and thus improving the quality of iPSC cultures.
StemCultures is developing a portfolio of DISC devices with StemBeads releasing other recombinant proteins as well as devices containing combinations of StemBeads releasing different growth factors and cytokines at specific concentrations. Custom StemBeads and DISC devices for specialized uses are also available.
Sally Temple, PhD, is president of StemCultures.

