A new antibiotic, isolated from bacteria that are unculturable, seems capable of combating harmful bacteria and even multi-resistant “superbugs.” The new drug, Clovibactin, efficiently killed drug-resistant Gram-positive bacterial pathogens. In addition, bacteria did not develop any detectable resistance.
This research is published in Cell in the paper, “A new antibiotic from an uncultured bacterium binds to an immutable target.”
Antimicrobial resistance is a major problem for human health and researchers worldwide are looking for new solutions. “We urgently need new antibiotics to combat bacteria that become increasingly resistant to most clinically used antibiotics,” said Markus Weingarth, PhD, associate professor from the chemistry department of Utrecht University.
Few new antibiotics have been introduced into the clinic over the last decades and they often resemble older, already known antibiotics.
“Clovibactin is different,” said Weingarth. “Since Clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance.”
Clovibactin was discovered by NovoBiotic Pharmaceuticals and microbiologist Kim Lewis, PhD, professor at Northeastern University. Earlier, they developed a device that allows the growth of unculturable bacteria. The majority (99%) of all bacteria are unculturable and could not be grown in laboratories previously, nor mined for novel antibiotics. Using the device, called iCHip, the U.S. researchers discovered Clovibactin in a bacterium isolated from a sandy soil from North Carolina: E. terrae ssp. Carolina.
The researchers showed that Clovibactin successfully attacks a broad spectrum of bacterial pathogens and successfully treated mice infected with Staphylococcus aureus.
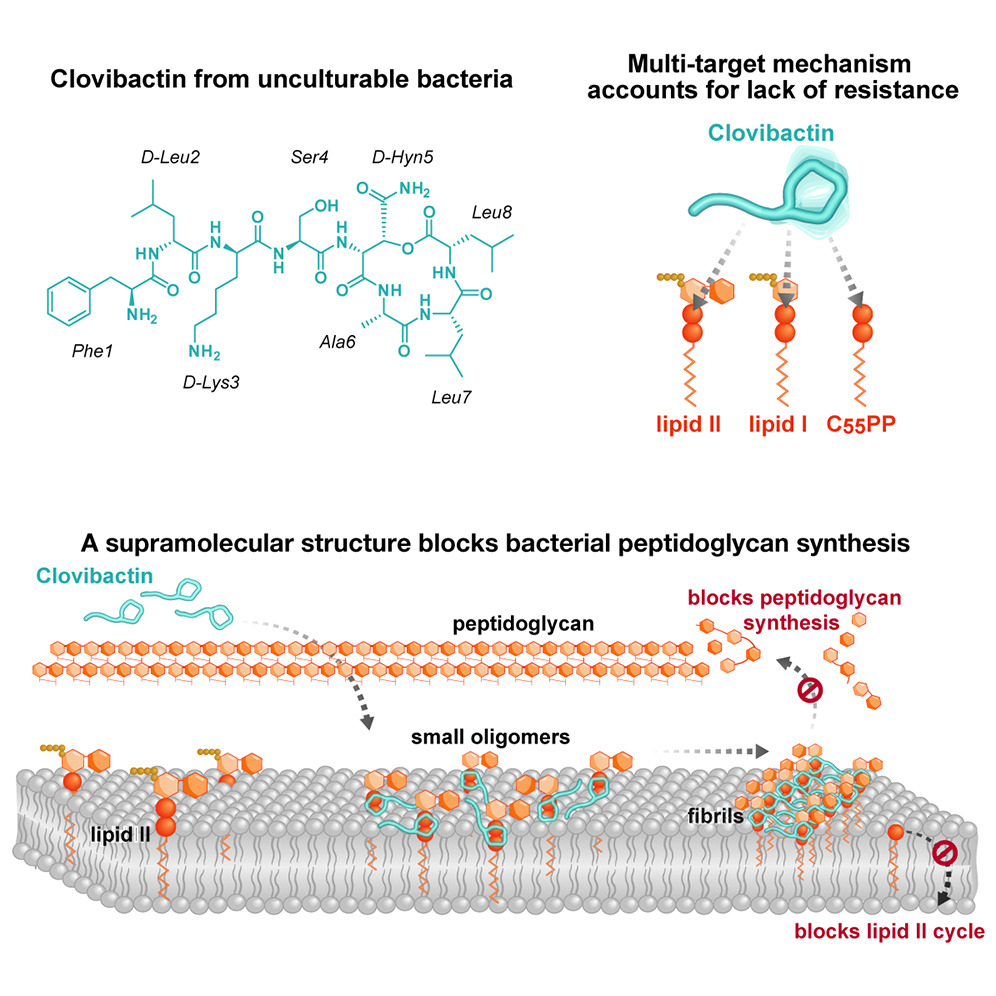
The researchers used biochemical assays, solid-state nuclear magnetic resonance, and atomic force microscopy, to dissect Clovibactin’s mode of action and found that it has an unusual killing mechanism. It targets not just one, but three different precursor molecules that are all essential for the construction of the cell wall.
More specifically, the authors wrote, “Clovibactin blocks cell wall synthesis by targeting pyrophosphate of multiple essential peptidoglycan precursors (C55PP, lipid II, and lipid IIIWTA). Clovibactin uses an unusual hydrophobic interface to tightly wrap around pyrophosphate but bypasses the variable structural elements of precursors, accounting for the lack of resistance.”
“The multi-target attack mechanism of Clovibactin blocks bacterial cell wall synthesis simultaneously at different positions. This improves the drug’s activity and substantially increases its robustness to resistance development,” said Tanja Schneider, PhD, professor of pharmaceutical microbiology at the University of Bonn in Germany.
“Clovibactin wraps around the pyrophosphate like a tightly fitting glove. Like a cage that encloses its target” said Weingarth. This is what gives Clovibactin its name, which is derived from Greek word “Klouvi,” which means cage. The remarkable aspect of Clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it ignores that variable sugar-peptide part of the targets. “As Clovibactin only binds to the immutable, conserved part of its targets, bacteria will have a much harder time developing any resistance against it. In fact, we did not observe any resistance to Clovibactin in our studies.”
Upon binding the target molecules, Clovibactin self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
“Selective and efficient target binding,” the authors noted, “is achieved by the sequestration of precursors into supramolecular fibrils that only form on bacterial membranes that contain lipid-anchored pyrophosphate groups.”
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why Clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Weingarth. “Clovibactin hence has potential for the design of improved therapeutics that kill bacterial pathogens without resistance development.”