By Kevin Davies, PhD

Mandy Boontanrart, PhD, a postdoc at ETH Zurich in Switzerland, has a particularly personal motivation for her research on a novel genetic therapy for sickle cell disease (SCD). She is a carrier of a mutation for another hemoglobinopathy, alpha thalassemia.
She initially received her diagnosis during a routine health checkup before entering high school in her native Thailand. Asked if she’s experienced any health issues, she casually responds, “I’ve never been a wild-type person, so I don’t know what that feels like!” She says she was always chosen last for sports teams because of her lack of stamina.
Her diagnosis became an inspirational “driving force,” especially after her younger brother fainted during a PE class. “I remember being very upset about this, I was quite certain it was because he was a carrier of this disease,” she told GEN. “I wanted to understand what was going on.”
Years later, enrolling in a PhD program at the University of California, Berkeley, she learned that the lab of Jacob Corn, PhD, had a project on SCD. “I just knew this was meant to be,” she said. After Corn moved to Switzerland five years ago, Boontanrart followed suit. The fruits of her research—an ingenious CRISPR-based strategy for treating SCD—were recently published in eLife. Boontanrart, Corn, and colleagues report some promising proof-of-principle results in boosting the expression of the delta globin gene, which is normally expressed in adults at very low levels.
“The concept of enabling transcriptional induction of delta globin is very creative and could nicely complement ongoing efforts to induce gamma globin to treat the hemoglobin disorders,” observes Vijay Sankaran, MD PhD, chair of the division of hematology/oncology at Boston Children’s Hospital (Harvard Medical School) and an associate member of the Broad Institute. Fifteen years ago, Sankaran’s research identifying a key molecular handbrake (BCL11A) of fetal globin gene expression sparked the most advanced gene editing strategy so far for SCD. But a major advantage compared to the gamma globin approach, Sankaran says, is that “the delta globin gene is poised for transcription, as it is very close to the actively produced adult beta globin gene, so this could be very advantageous as this strategy is explored further.”
Delta difference
There are two forms of hemoglobin (Hb) in adults: about 97 percent of Hb (HbA) is made up of two alpha chains paired with two beta chains. In HbA2, which makes up less than three percent of the total Hb protein, the alpha chains pair with a pair of delta globin chains. The delta globin gene sits upstream of the closely related beta globin gene in a gene cluster on chromosome 11.
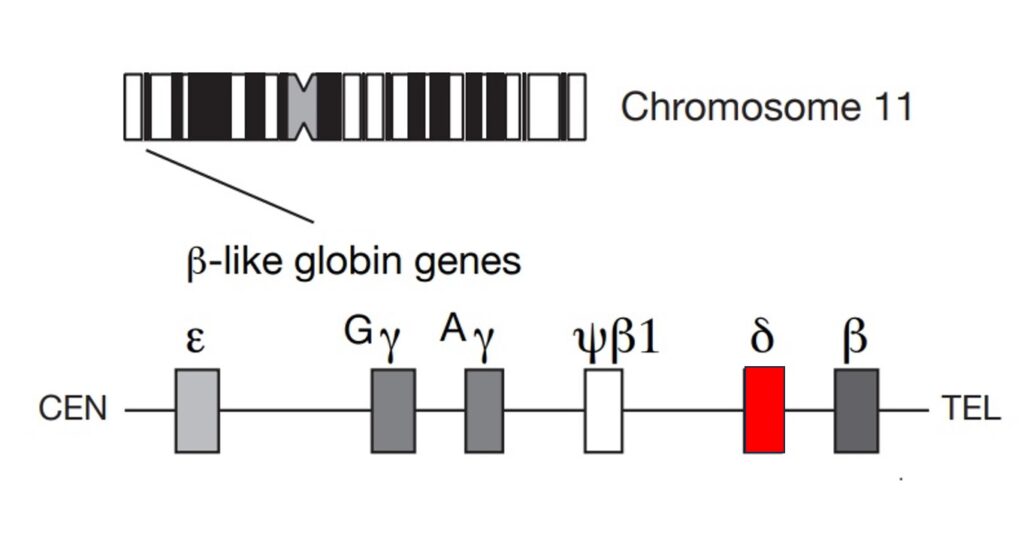
In humans, the delta globin is poorly expressed because the gene promoter is partially broken—missing crucial transcription elements to drive transcription. Interestingly, in dogs, it is the delta globin gene that is predominantly expressed, while the canine beta gene was silenced during evolution. “During evolution,” Boontanrart says, “the cells just thought, we don’t need two copies of this very similar globin gene and silenced one.”
Several biotech companies are making rapid strides in the clinic with a strategy that upregulates fetal (gamma-) globin expression to compensate for the faulty beta chains in SCD containing the causative mutation. Launched in 2019, the exa-cel trial sponsored by Vertex Pharmaceuticals and CRISPR Therapeutics has produced impressive results and will come up for regulatory approval in the next few months. Several other biotech companies, including Beam Therapeutics and Editas Medicine, are working on similar strategies using genome editing.
Boontanrart is pursuing a related but surprisingly novel idea. She got the idea of upregulating delta globin, rather than gamma globin, while delving into the literature writing her PhD thesis. Some earlier studies in the B.C. (“before CRISPR”) era using transgenic models had considered upregulating the delta globin idea but there was no way to modulate expression of the endogenous gene.
“It seemed like a very important thing to try,” she said, “because [raising] fetal hemoglobin has shown so many benefits for these diseases. But delta globin could be as good, if not better, because it is more identical” to the beta globin gene. (The delta gene sequence is 93 percent homologous to the beta globin, whereas the gamma globin gene is only 76 percent homologous.)
Initially, Boontanrart was disappointed that using CRISPR-Cas9 to engineer a binding site for a single potent transcription factor—KLF1—did little to boost delta globin expression. “Then I realized, maybe this is why there haven’t been any papers published about delta globins, because people have tried it, but they didn’t try to put enough transcription factors back in.”
Previous studies showed that the delta globin upstream region contains mutations in a series of promoter sequences, including those for transcription factors KLF1, NF-Y, and TFIIB. Using CRISPR-Cas9 to reconstitute those sequences to resemble their counterparts upstream of the beta globin gene, Boontanrart’s team was able to raise HbA2 expression in a cell line more than tenfold over the wild-type levels in unedited cells. “To our knowledge,” the authors write, “this is the first genomic editing of the [delta globin] promoter that results in increased HbA2” production.
“Assuming [HbA2] has the same stability and oxygen affinity as HbA, then I don’t see any reason why upregulating it wouldn’t work,” commented Samuel E. Lux IV, MD, Distinguished Professor of Pediatrics at Boston Children’s Hospital/Harvard Medical School. “On the other hand, I don’t see any reason why it is a better strategy that upregulating HbA or F.”
Path ahead
Boontanrart and her colleague Jan Nelis are now leading a team of five researchers building on the results of the eLife study, including testing different spacing of the transcription factor-binding sites, as well as various NHEJ (non-homologous end-joining) pathway inhibitors to increase the knock-in efficiency. She hopes to begin studies on patient cells shortly as well.
Although there is a long road ahead, Boontanrart has a clear vision. The first priority is to develop an ex vivo approach “because this process is a lot more established, working, showing good results and safe.” But ex vivo therapy is, she says, “personalized medicine, which is great but makes it very expensive if we want to be able to reach patients who need it.” She adds: “In vivo gene editing is the future of gene editing.”
With recent progress in developing such in vivo methods of gene editing, Boontanrart hopes that, by the time her group has made clinically relevant progress ex vivo, “in vivo companies would be far enough along that we would be able to partner and go forward that way.” That won’t happen for several years, she acknowledges.
Together with Nelis, Boontanrart has co-founded “a little start-up” called Ariya Bio. “This is the product of many research grants that we’ve received from [Swiss government] and also ETH Zurich. The lab is in the incubator lab space of ETH.
The name “Ariya” has a Sanskrit origin. “It means to be enlightened or to understand,” Boontanrart says. “I love this meaning.”

