Salk Institute scientists discovered a relationship between killer T cell exhaustion and the body’s sympathetic—“fight-or-flight”—stress response. The research showed that beta-blockers could inhibit the interaction between these CD8+ T cells and sympathetic stress response hormones binding to the ADRB1 receptor, enabling the killer T cells to fight the tumor more efficiently. The study demonstrated the potential benefits of pairing beta-blockers with existing immunotherapies to improve cancer treatment by boosting killer T cell function.
The findings establish a new link between the sympathetic stress response and how the immune system responds to cancer. They also demonstrate the potential benefits of pairing beta-blockers with existing immunotherapies to improve cancer treatment by bolstering killer T cell function.
“There is no question immunotherapy has revolutionized cancer patient treatment—but there are many patients for whom it’s ineffective,” said Susan Kaech, PhD, director of Salk’s NOMIS Center for Immunobiology and Microbial Pathogenesis, and senior author of the team’s published paper in Nature. “Finding that our nervous system can suppress the function of cancer-destroying immune cells opens up entirely new ways to think about how to rejuvenate T cells in tumors.”
Kaech and colleagues described their study in a report titled “The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion,” in which they noted, “our results establish a connection between the sympathetic stress response, tissue innervation and T cell exhaustion… we uncover a new mechanism by which blocking β-adrenergic signaling in CD8+ T cells rejuvenates anti-tumour functions.”
CD8+ T cells are essential components of the immune response against viral infections and tumors, and are capable of eliminating infected and cancerous cells, the authors noted. However, even for the immune system’s specialized killer T cells, seeking and destroying cancer cells around the clock can be exhausting. “T cell exhaustion is a specific CD8+ T cell differentiation state that is induced by chronic exposure to antigen such as from chronic viral infections or cancer,” the investigators wrote. If scientists can understand why killer T cells become exhausted, then they can create more resilient cancer-killing cells.
And while it’s known that chronic antigen exposure contributes to CD8+ T cell exhaustion less is known about how stress responses in tissues regulate T cell function. For their study the researchers focused on sympathetic nerves that innervate our organs and produce the messenger hormone noradrenaline, which is also a stress hormone. The sympathetic nervous system is responsible for mediating the body’s stress response, also known as the fight-or-flight response.
The scientists used a variety of cancer and chronic illness models in mice and human tissue samples to study when and how killer T cells are influenced by the sympathetic nerves. They found that the sympathetic nerves were producing noradrenaline, which was binding to killer T cells using a receptor called ADRB1. Exhausted CD8+ killer T cells expressed more ADRB1 receptors than their functional counterparts, allowing the T cells to “listen” to the noradrenaline released by the nerves.
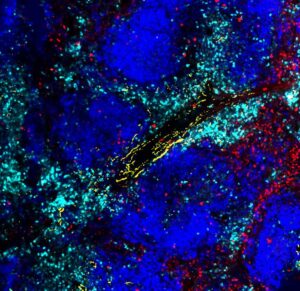
The authors also found that the exhausted T cells do not just listen to nerves from afar, but cluster right around them in tissues. “Exhausted CD8+T cells cluster around sympathetic nerves in an ADRB1-dependent manner,” they noted. Surprisingly, the ADRB1 receptor provided the T cells with critical instructions to migrate near the nerves, which in turn suppressed their functions—making them worse at fighting cancer.
Given the high expression of ADRB1 by the exhausted tumor-infiltrating lymphocytes (TILs), the authors wanted to test whether intercepting this noradrenaline and ADRB1 interaction could prevent killer T cell exhaustion and have a positive impact on the ability of the killer T cells to destroy cancer cells. The team did this either by knocking out the ADRB1 gene, or by impairing ADRB1 function using beta-blockers. Results from gene knockout tests suggested that the ADRB1 knockout cells impaired their migration towards the sympathetic nerves, and that the cells responded more strongly to immune checkpoint blockade (ICB) immunotherapy than wild-type cells. Experiments in which they then used beta-blockers to block the ARBD1 receptor in chronically infected mice resulted in more-functional killer T cells that were better at destroying cancer cells. “Taken together, these findings show that pharmacological blockade of ADRB1 prevents functional exhaustion of antigen-specific CD8+ T cells and highlight a new adjuvant therapy for patients with chronic infections,” the investigators stated.
Encouragingly, experiments showed that tumors were significantly smaller in melanoma-bearing mice treated using beta blocker therapy in combination with anti-PD-1 plus anti-CTLA-4 immunotherapy. “Ablation of β1-adrenergic signalling limits the progression of T cells towards the exhausted state in chronic infection and improves effector functions when combined with immune checkpoint blockade (ICB) in melanoma,” the investigators stated. And in summary, they wrote, “Here we propose that the adrenergic receptor ADRB1 is a new immune checkpoint with increased expression on exhausted T cells … Genetic or pharmacological blockade of ADRB1 with existing clinically approved drugs (β-blockers) prevented terminal exhaustion differentiation of T cells and increased T cell function in chronic viral infection and in a melanoma model.”
“The innervation of tumors is an understudied area of tumor immunology,” said first author Anna-Maria Globig, a postdoctoral researcher in Kaech’s lab. “Our study has now uncovered that nerves contribute to the process of T cell exhaustion in tumors, where T cells become worn out and less powerful in their fight against the tumor over time. If we can unravel the details of how nerves suppress the body’s immune response to cancer and why the exhausted T cells move towards the nerves, we can begin to target this process therapeutically.”
According to Kaech, the researchers hope to expand their understanding of the exhausted killer T cell environment to learn more about why stress makes us sicker. “We were able to find a new pathway that we can target with beta-blockers to create more resilient killer T cells that resist exhaustion and fight cancer better,” said Globig.
Since beta-blockers are already clinically used, the team hopes to implement their proposed cancer-fighting regimen soon in patients with lung cancer. By partnering with clinicians, they hope to study more human cancer tissue samples to enrich their findings and provide further evidence of the efficacy of beta-blockers in cancer treatment. “Our findings provide a new framework for the regulation of antiviral and antitumor T cell responses by the sympathetic nervous system and support further investigation into targeted manipulation of adrenergic receptors in combination with ICB as a potential means to induce effective antitumor T cell responses,” the team concluded.


