Transmembrane proteins from the House of Nature are abundant, but they’re all off the rack. Yes, they have been tailored to serve diverse functions, from transmembrane transport to cell signaling, but they are hard to alter. Transmembrane protein knockoffs might offer more flexibility. Bespoke transmembrane proteins might be even better, dressing up cells so that they take on entirely new functions.
Few transmembrane proteins, however, have come from protein designers. A zinc-transporting tetramer called Rocker has been engineered, as well as an ion-conducting oligomer, which emulates a bacterial design. These encouraging examples notwithstanding, transmembrane protein form/function relationships are still hard to grasp. Unlike proteins that operate in the watery solution that make up the cells' cytoplasm or in the extracellular fluid, transmembrane proteins embed themselves within the cell membrane. Actually, complex transmembrane proteins weave in and out of the cellular membrane, passing into and out of the membrane’s oily interior.
Predicting how a large, multipass transmembrane protein design might fold into shape and function while spanning such different environments has been challenging. Yet the challenge has been taken up by scientists based at the University of Washington Institute for Protein Design.
In a new study, a team of protein designers led by Dean Baker, Ph.D., used a computer program called Rosetta. This program, which was designed in Dr. Baker’s lab, can predict the structure a protein will fold into after it has been synthesized. In the new study, Rosetta was used to design transmembrane monomers, homodimers, trimers, and tetramers with 76- to 215-residue subunits containing two to four membrane-spanning regions and up to 860 total residues that adopt the target oligomerization state in detergent solution.
Additional details appeared March 2 in the journal Science, in an article entitled “Accurate Computational Design of Multipass Transmembrane Proteins.”
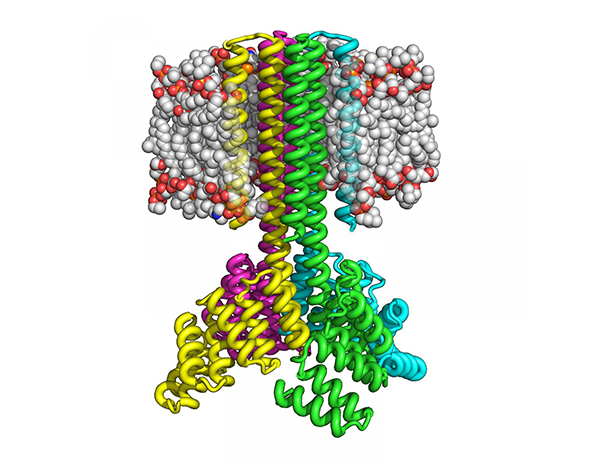
“The designed proteins localize to the plasma membrane in bacteria and in mammalian cells, and magnetic tweezer unfolding experiments in the membrane indicate that they are very stable,” the article’s authors reported. “Crystal structures of the designed dimer and tetramer—a rocket-shaped structure with a wide cytoplasmic base that funnels into eight transmembrane helices—are very close to the design models.”
A protein's shape forms from complex interactions between the amino acids that make up the protein chain and between these amino acids and the surrounding environment. Ultimately, the protein assumes the shape that best balances out all these factors so that the protein achieves the lowest possible energy state.
The Rosetta program can predict the structure of a protein by taking into account these interactions and calculating the lowest overall energy state. It is not unusual for the program to create tens of thousands of model structures for an amino acid sequence and then identify the ones with lowest energy state. The resulting models have been shown to accurately represent the structure the sequence will likely assume in nature.
In aqueous fluids, amino acid residues that have polar sidechains—components that can have a charge under certain physiological conditions or that participate in hydrogen bonding—tend to be located on the surface of the protein where they can interact with water, which has negatively and positively side charges to its molecule. As a result, polar residues on proteins are called hydrophilic, or “water-loving.”
Nonpolar residues, on the other hand, tend to be found packed within the protein core away from the polar aqueous fluid. Such residues are called hydrophobic or “water fearing.” As a result, the interaction between the water-loving and water-fearing residues of the protein and the surrounding watery fluids helps drive protein folding and stabilizes the protein's final structure.
In membranes, however, protein folding is more complicated because the lipid interior of the membrane is nonpolar, that is, it has no separation of electrical charges. This means to be stable the protein must place nonpolar, water-fearing residues on its surface and pack its polar, water-loving residues inside. Then it must find a way to stabilize its structure by creating bonds between the hydrophilic residues within its core.
According to Peilong Lu, Ph.D., a senior fellow in the Baker lab and the lead author of the Science paper, the key to solving the problem was to apply a method developed by the Baker lab to design proteins so that the polar, hydrophilic residues fit in such a way that enough of them would form polar–polar interactions that could tie the protein together from within.
“Putting together these 'buried hydrogen bond networks' was like putting together a jigsaw puzzle,” Dr. Baker explained.
With this approach, Dr. Lu and his colleagues were able to manufacture the designed transmembrane proteins inside bacteria and mammalian cells by using as many as 215 amino acids. The resulting proteins proved to be highly thermally stable and able to correctly orient themselves in the membrane. Like naturally occurring transmembrane proteins, the proteins are multipass, meaning they traverse the membrane several times, and assemble into stable multiprotein complexes, such as dimers, trimers, and tetramers.
“We have shown that it is now possible to accurately design complex, multipass transmembrane proteins that can be expressed in cells. This will make it possible for researchers to design transmembrane proteins with entirely novel structures and functions,” asserted Dr. Lu.