Researchers at the Francis Crick Institute and UCL Queen Square Institute of Neurology, working with Faculty AI, have shown how machine learning can accurately predict subtypes of Parkinson’s disease (PD), by using images of patient-derived stem cells.
The team used stem cell technology to derive control or patient-derived neurons, and generated four different disease subtypes. They then created machine learning models that could both predict presence of Parkinson’s disease, and accurately classify the four disease subtypes with an accuracy of up to 95 percent. This capability, the team suggests, could pave the way to the development of strategies for personalized medicine and targeted drug discovery.
James Evans, a PhD student at the Crick and UCL, is co-first author of the team’s published paper in Nature Machine Intelligence, titled “Prediction of mechanistic subtypes of Parkinson’s using patient-derived stem cell models.” In their report the scientists concluded, “Altogether, we show that machine learning approaches applied to patient-derived cells are highly accurate at predicting disease subtypes, providing proof of concept that this approach may enable mechanistic stratification and precision medicine approaches in the future.”
Parkinson’s disease is a neurodegenerative condition impacting movement and cognition. Symptoms and disease progression vary from person to person due to differences in the underlying mechanisms causing the disease. “The age of onset, rate of disease progression, and severity of motor and non-motor symptoms display considerable individual variation,” the authors noted. “This is most likely due to differences in the underlying molecular mechanisms occurring in different subtypes of the disease.
Parkinson’s disease is associated with misfolding of key proteins, and dysfunction in the clearance of faulty mitochondria, the source of energy production in the cell. The majority of Parkinson’s disease cases start sporadically, but some can be linked to genetic mutations. But until now there hasn’t been a way to accurately differentiate subtypes, which means people are given nonspecific diagnoses and don’t always have access to targeted treatments, support or care.
“Critically, there are currently no approaches to define the molecular heterogeneity and therefore no opportunity to understand the mechanisms that may drive the different phenotypic subtypes,” the authors added. “An unmet challenge is to make an early and accurate molecular-level diagnosis of the condition, as this would enable the field to consider targeted interventions appropriate to an individual’s condition, and offer an opportunity to do this at the earliest possible time.”
For their newly reported study the team set out to apply a deep learning approach to human cellular models of Parkinson’s disease, to generate predictive models of different disease mechanisms. They generated patient-derived induced pluripotent stem cell (iPSC)-derived cortical neurons, and chemically created four different subtypes of Parkinson’s disease, two involving pathways leading to toxic build-up of α-synuclein (α-Syn ) protein, and two involving pathways leading to faulty mitochondria, so creating effectively a ‘human model of brain disease in a dish’.
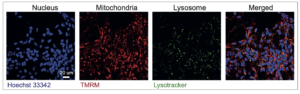
The researchers then fluorescently labelled specific cellular compartments–nucleus, mitochondria and lysosomes– and carried out high-content live single-cell imaging of the iPSC-derived neurons. Using data from more than 1.5 million cells, they generated models to predict disease state and subtypes.
There were two broad types of classifier. One was a prediction classifier, based on 56 automatically extracted features, which could carry out deep profiling of these cellular phenotypes. “… this classifier has the advantage of high explainability using the ranking of features,” the team noted. The second type of prediction classifiers were based on images and convolutional neural network (CNN) analyses, which used the power of computer vision to take from the images a large amounts of unbiased information. “ … this classifier has very high accuracy but less explainability,” the authors said. The results showed that the CNN-based image classifiers were able to correctly classify images to accurately identify diseased from healthy states, with high performance, “achieving close to 80–100% accuracy for different disease states.”
Their findings confirmed that the mitochondria and lysosomes were the most important features in predicting the correct subtype–supporting the involvement of these components in how Parkinson’s disease develops. But the study did also find that other cell compartments, including the nucleus were also important, and there were in addition other aspects of the images that have yet to be explained.
The team suggested that their approach offers advantages over traditional image analysis methods, which can quantify well-defined structural properties, but can’t capture all the information contained within the imaging data. Using traditional image processing software, researchers will commonly have to choose which feature (or features) to combine to quantify from a vast array of possible cellular phenotypes, they noted. “ … this is challenging, time-consuming and may be subject to bias.” In contrast, machine learning can decipher cellular features in an unbaised manner, and with higher accuracy. “Our approach is well placed as a preclinical platform to have high predictive value for disease as it is a human model of brain disease in a dish that captures live information on the two critical organelles implicated in PD,” the team commented.
Evans added, “Now that we use more advanced image techniques, we generate vast quantities of data, much of which is discarded when we manually select a few features of interest. Using AI in this study enabled us to evaluate a larger number of cell features, and assess the importance of these features in discerning disease subtype. Using deep learning, we were able to extract much more information from our images than with conventional image analysis. We now hope to expand this approach to understand how these cellular mechanisms contribute to other subtypes of Parkinson’s.”
The authors further concluded, “Importantly, as PD is highly heterogeneous, this platform may enable the disease mechanism in patient cells to be classified. This may have significant clinical implications in both diagnosis and treatment, as the identification of cellular mechanisms may indicate their likely response to proteinopathy (for example, targeting α-Syn) versus mitochondrial (for example, antioxidant therapy) treatments.”
Co-corresponding author Sonia Gandhi, PhD, assistant research director and group leader of the Neurodegeneration Biology Laboratory at the Crick, said, “We understand many of the processes that are causing Parkinson’s in people’s brains. But, while they are alive, we have no way of knowing which mechanism is happening, and therefore can’t give precise treatments.
“We don’t currently have treatments which make a huge difference in the progression of Parkinson’s disease. Using a model of the patient’s own neurons, and combining this with large numbers of images, we generated an algorithm to classify certain subtypes—a powerful approach that could open the door to identifying disease subtypes in life. Taking this one step further, our platform would allow us to first test drugs in stem cell models, and predict whether a patient’s brain cells would be likely to respond to a drug, before enrolling into clinical trials. The hope is that one day this could lead to fundamental changes in how we deliver personalized medicine.”
Co-author James Fleming, PhD, chief information officer at the Crick, who worked with Faculty AI on the project, said, “AI is a fascinating and powerful technology, but one which is often rendered impenetrable by hype and jargon. This paper came about as a result of a unique industry partnership with Faculty to see if a group of complete AI beginners could learn and apply best practice directly to their science in a very compressed time frame. The success of this project not only proved that they could, unlocking new insights in the process, but has also helped drive investment into the rapid expansion of our own AI and software engineering team, which has over 25 projects ‘in-flight’ with different labs across the Crick, with new projects kicking off every month.”
The next steps for the research team are to understand disease subtypes in people with other genetic mutations, and to work out whether sporadic cases of Parkinson’s disease (i.e., without genetic mutations) can be classified in a similar way.



