Once an ecosystem is disturbed, restoring it can be difficult. And when the disturbed ecosystem is a patient’s microbiome, restoring the patient to health can be even more difficult. Just one ecosystem element that proliferates or diminishes beyond bounds may throw multiple elements into disarray, creating a dysbiosis that resists simple remedies.
Because a patient’s microbiome consists of interacting elements—including elements that extend beyond the microbiome itself—these elements cannot be seen in isolation. Rather, they are dynamic parts of a systemic whole. Touch any one of them, and the effects of doing so may ripple outward in unpredictable ways—unless the elements and their interactions are thoroughly understood.
Fortunately, comprehensive knowledge of the microbiome has been and continues to be accumulated by researchers. Even better, this knowledge is being exploited in clinical applications. As this article shows, these applications are being developed by firms of various types—biotechnology companies, contract research organizations, and personal care companies.
Antibodies, not antibiotics
“We are covered by, and protected by, and interacted with by vast microbial ecosystems,” says Julius Goepp, MD, founder of Scaled Microbiomics. Everywhere the body comes into contact with the outside environment, you’ll find a thriving community of microbes. This includes places that are obviously “external”—like skin and hair (including the skin and hair of underarms and nostrils)—as well as places that we consider to be “internal,” like the gastrointestinal tract.
“The surface of our gut is continuous with the outside world,” Goepp points out. The miracle of our gut, he continues, is that it can transport “two pounds of very nasty material [while keeping] it one cell layer away from our precious, sterile, inside tissue.”
But one cell layer can prove precarious protection, especially if that microbial ecosystem gets out of balance. The gut’s microbial ecosystem, Goepp suggests, is like the Amazon rainforest ecosystem in that it is resilient, but only to a point. When subjected to stressors such as prolonged exposure to antibiotics, a diet high in certain additives and low in fiber, and environmental pollution, the microbial ecosystem can be tipped far enough out of balance that a new normal becomes established. This is called dysbiosis, and it’s increasingly linked with a number of noncommunicable diseases, such as diabetes, neurodegenerative diseases, and cancer.
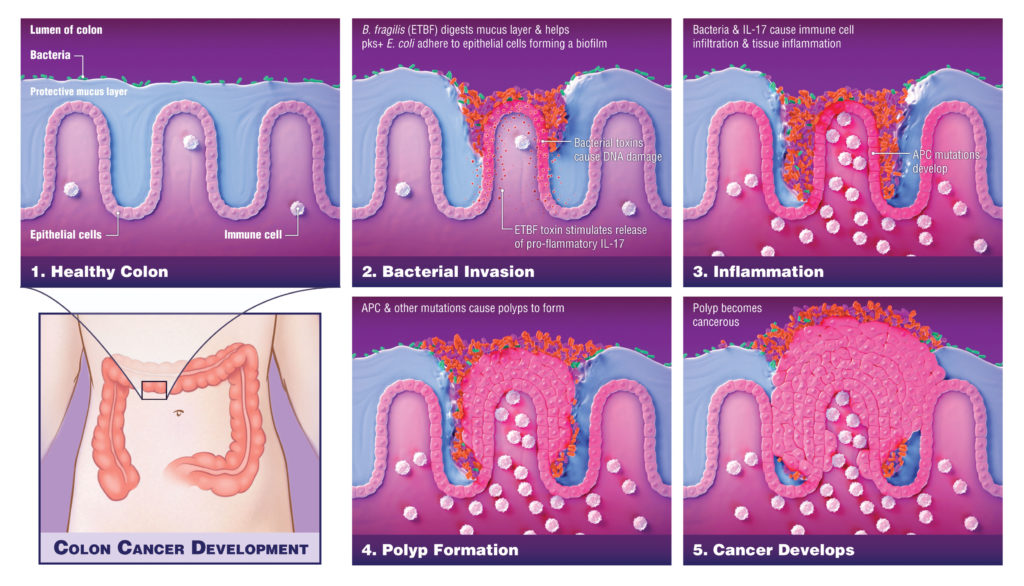
For instance, hereditary colorectal cancer has been linked to an excess of two damaging types of bacteria. The first, a strain of Bacteroides fragilis, attacks the protective layer separating the microbiome from the colon cells. The second is a type of Escherichia coli that produces a DNA-damaging metabolite called colibactin. When B. fragilis breaks down the protective layer, E. coli may gain access to the intestinal epithelial cells, and colibactin may wreak havoc with the DNA.
In a retrospective longitudinal study of colorectal cancer patients, the telltale signature of colibactin-induced mutations was found in 20% of tumors. Evidence suggests that most of that damage was sustained during childhood. “Had there been an intervention to stop that genomic damage from happening, those people might not have had their cancer,” Goepp suggests.
Goepp has high hopes for the antibody approach. “We’re applying an old, established, mature technology to a new organ, a new series of cell types, and a new series of signaling molecules,” he says. “We believe that we can use our methodology to learn how disease cascades work and how they can be disrupted.”
Human-first discovery
“The microbiome is a new organ that we discovered just 12 to 15 years ago,” says Sonia Timberlake, PhD, vice president of research for Finch Therapeutics. “There’s a lot of complex and unknown biology.”
To tackle that complex biology, Finch is taking a human-first approach that draws on years of data from fecal microbiota transplants. The company is leveraging its long-standing association with OpenBiome, the first public stool bank, to move beyond fecal transplants and begin delivering full-spectrum microbiota in pill form. Two of OpenBiome’s founders, Mark Smith, PhD, and Zain Kassam, MD, are also founders of Finch, and now serve as Finch’s chief executive officer and chief medical officer, respectively.
OpenBiome was founded to expand access to fecal transplants for the treatment of Clostridioides difficile overgrowth, which can cause debilitating inflammation in the gastrointestinal tract. A naturally occurring component of the microbiome, C. difficile can get out of hand because it grows back faster than other species after a course of antibiotics. Once established, C. difficile can prove extremely hard to unseat, even with more antibiotics. A fecal transplant reintroduces a diverse population of naturally occurring gut bacteria, and in theory, once antibiotics are stopped, these bacteria can resume their normal abundances and keep C. difficile in check.
In addition to producing full-spectrum microbiota products, Finch is developing “rationally selected microbiota” products, a strategy the company hopes will enable it to scale up the technology. “Because each healthy donor’s community is slightly different, and each patient’s community before and after treatment is slightly different, you can start to ask which microbes are the active ingredients,” Timberlake explains. A full community transplant contains approximately 500 different microbial strains, but only 50 to 100 of them might take hold in the recipient. Using high-throughput DNA sequencing and metabolomics, scientists at Finch can identify which species correlate with the patients who get better.
Identifying the species most important for healing the gut is a key step in scaling up production. Instead of extracting a full community of microbes from an individual donor for every patient, a laborious process, investigators can grow and develop rationally selected strains into a more streamlined, off-the-shelf product. “Your product is much simpler, and you can really understand the safety at a gene-by-gene level,” Timberlake points out. She adds, however, that few established industrial protocols work well for culturing human gut bacteria, and growing them at scale remains a major technical challenge.
Based on the success of microbiome transplants for treating C. difficile, Finch hopes to develop treatments for inflammatory bowel disease (IBD), an autoimmune disease that causes inflammation of the gastrointestinal tract. IBD includes Crohn’s disease and ulcerative colitis, and it can be managed with medications or surgery. These treatments, which can involve powerful immune suppressants, are often expensive and unpleasant, and they work only in a fraction of patients.
“This field has so much promise to treat a lot of diseases increasing in our population,” Timberlake maintains. “I really think these therapies are going to be very safe, and they have a lot of potential.”
Skin deep
Like the gut, the skin hosts a teeming community of microbes, but studies of this microbiome began much more recently. Skin dysbiosis has been correlated with some skin disorders, such as atopic dermatitis (AD) and dandruff. Researchers are working to understand exactly which microbes might be involved, and whether treatments directed at those microbes can lessen the symptoms.
AD is a common, allergy-related skin inflammation that usually begins in childhood. Babies who are born by cesarean section are at increased risk for AD, explains Cécile Clavaud, PhD, project leader, Skin Microbiome Unit, Research and Innovation, L’Oréal. These babies have a very different community of microbes on their skin than babies born vaginally.

The typical treatment for AD involves corticosteroids to decrease the itching and redness associated with a flare-up. “This has a short-term effect,” says Clavaud. “The redness decreases but comes back a few weeks later.” She notes that watching changes in the microbiome over time reveals an overgrowth of Staphylococcus aureus bacteria in the days leading up to an outbreak of AD. “During the crisis, you have much lower diversity,” she continues. “You have increasing S. aureus, and other bacteria decrease. One type of bacteria is taking all the space, all the food, everything.”
L’Oréal researchers demonstrated that applying an emollient containing a probiotic extract could extend the time between AD outbreaks. “This extract will stimulate innate immunity and help the microbiome to recover its right diversity,” Clavaud asserts. The inactivated probiotic agent stimulates production of certain cytokines by immune cells in the skin. These not only help keep S. aureus in check, they also have anti-inflammatory activity that reduces the redness and itching characteristic of AD.
In their studies of the scalp, scientists at L’Oréal have shown that people with dandruff have a disruption in the ratio of two bacteria. A healthy scalp has more Propionibacterium acnes and less Staphylococcus epidermidis, while in dandruff sufferers, the ratio is reversed.
Dandruff is typically associated with an excess of yeast in the Malassezia genus, and over-the-counter shampoos that treat dandruff generally contain antifungal agents. L’Oréal is working on a formula specifically targeting S. epidermidis. “By targeting the bad bacteria, we limit its growth, and there’s an additional improvement in the symptoms,” Clavaud maintains.
Our understanding of the skin microbiome is only just beginning. Clavaud and her colleagues recently published the genome sequence of Malassezia restricta to better understand its role in the scalp community and how it may contribute to dandruff. They are also working to uncover the roles of environmental pollution and aging on the microbiome. “The story is continuing,” declares Clavaud.
Ultra-high-resolution genomic profiling
When Clinical Microbiomics set up shop in 2015, it began by offering 16S gene sequencing for microbiome samples. It wasn’t long, however, before the company’s clients began requesting more advanced genomic analysis.
“Most of our clients are not bioinformaticians,” says H. Bjørn Nielsen, PhD, chief scientific officer of Clinical Microbiomics. To serve those clients better, Clinical Microbiomics provides various individualized services at different stages of a project’s development. “We have built a large framework of different bioinformatics tools,” Nielsen points out, and that framework enables the company to provide a powerful analysis specific to the project at hand. “It’s a very custom, tailored analysis that we’re providing,” he asserts.
Some clients, for instance, request guidance even before beginning the study, to determine an appropriate number of samples to include for the best chance of seeing an effect. Once samples have been collected, Clinical Microbiomics extracts and sequences DNA and applies bioinformatics tools to extract meaning from the data.
In 2014, Nielsen and colleagues at the Technical University of Denmark pioneered a technique called shotgun metagenomics. The method allows identification of new microbial species without the need for reference sequences, based on identification of co-abundant genes. Using shotgun metagenomics, Clinical Microbiomics provides ultra-high-resolution microbiome analysis to accurately detect differences in microbes of the same species.
Ultra-high-resolution analysis reveals each person’s microbiome as a genetically unique entity. Two people can harbor the same bacterial species, but each one’s population of that species could be genetically distinct from the other. “Then you have n of 1,” Nielsen explains. “So, how do you do any statistics with that?” Clinical Microbiomics uses statistical methods to construct phylogenetic trees of all these genetically distinct bacterial populations. By doing this, it may be possible to associate one branch of the tree with a particular host characteristic. “We have found many cases of this already, where there are clades of bacteria that are associated with a human phenotype,” Nielsen notes.
Another approach is to look at functional differences, rather than taxonomic ones. By identifying species that encode particular biochemical pathways, Clinical Microbiomics can uncover clues to possible mechanisms driving certain phenotypes.
“One of the new things we have started offering also is absolute abundances,” Nielsen says. “This is a bit of a game changer for certain types of analysis.” Relative abundances can be misleading, he points out, because a species that appears to be rising in abundance may actually be staying the same, while other species are dying off. Absolute abundances can provide critical information for understanding the role of microbiome metabolites in certain conditions.
Clinical Microbiomics aims to help academic or pharmaceutical researchers make sense of their data in the context of clinical applications. “There are lots of very good companies that have a clinical focus,” Nielsen relates. “They just want to understand how the microbiome works, and they don’t have a team of bioinformaticians for that. We can help them with that, so they can have their focus on the medical side.”

