Neurological conditions are famously hard to treat. Many develop in the brain, which is shielded by the skull against surgical intervention, and by the blood-brain barrier against pharmacological remedy. The brain also cloaks itself in architectural, chemical, and electrical complexities. Perhaps even more obscure than the brain’s internal workings, or dysfunctions, are the relations, or disharmonies, between the brain and other systems, such as the immune system and the microbiome.
To bring the nervous system’s complexities into focus and discern openings for new treatments, new technologies are being developed. For example, optogenetics technology is refining our view of the brain’s structure and function, and new blood tests may allow us to glimpse Alzheimer’s disease in patients a decade before the first symptoms appear. Such tools may bring rapid progress against neurological disease.
Diagnosis: Alzheimer’s disease
Even simply diagnosing neurological disorders such as Alzheimer’s disease is a challenge. Because Alzheimer’s disease symptoms overlap significantly with other dementias, a decisive diagnosis depends on expensive and invasive procedures. Positron emission tomography (PET) imaging can reveal the characteristic amyloid protein deposits on the brain, and cerebrospinal fluid (CSF) testing can track biomarkers. But for large-scale screening, nothing would beat a blood test.
This year, a particularly promising blood biomarker candidate emerged: pT217, or tau protein phosphorylated at position 217. Blood measurements of pT217 correlate extremely well with its levels in CSF and with the PET imaging of tau pathology in the brain. Plus, it’s nearly undetectable in people who don’t have Alzheimer’s.
“I don’t think we’re going to get much better than this for Alzheimer’s disease,” says Nicholas Ashton, PhD, a neuroscientist at the University of Gothenburg, Sweden, who was not involved in the study. “The next step is that it needs to be on an automated platform.”
A pT217 blood test that uses mass spectrometry has been developed at the Washington University School of Medicine in St. Louis and licensed by C2N Diagnostics. The company is hard at work developing a commercial assay. “[We have] strong expertise in taking an academic-grade assay like that and industrializing it,” says Joel Braunstein, MD, co-founder and CEO of C2N Diagnostics. “We have a roadmap based on our experience of optimizing an existing assay that is about to reach the clinic.”
In addition to improving throughput and lowering costs, the team seeks to characterize how the biomarker behaves in different populations, including different racial and ethnic groups and people who may have additional health conditions.
Scientists at C2N have spent the last few years developing a mass spectrometry assay that reliably measures the ratio between two peptides in the blood, amyloid beta 42 and amyloid beta 40, an indicator that the amyloid plaques responsible for Alzheimer’s disease have begun to form in the brain.
“We now understand,” Braunstein asserts, “how those analytes behave, in the presence of different conditions and in different populations, and that test will soon be on the market.” He adds that this understanding will serve the company well as it works to optimize the pTau217 assay for widespread use.
Targeting the microbiome to protect the brain
In the early 1800s, when James Parkinson first described the “shaking palsy” that would later bear his name, he noted that in addition to tremors and muscle weakening, his patients also suffered with constipation. Curiously, digestive symptoms often precede the onset of motor symptoms, and over the last two decades, researchers have begun to suspect that Parkinson’s originates in the digestive system.

“Fast forward a couple hundred years, and now we have identified that diseases like Parkinson’s and others can emanate via the enteric nervous system,” says David H. Donabedian, PhD, co-founder and CEO of Axial Biotherapeutics. The enteric nervous system (ENS) encompasses the neurons that serve the digestive tract, and it is connected to the brain via the vagus nerve.
Sarkis Mazmanian, PhD, professor of microbiology at the California Institute of Technology and co-founder of Axial Biotherapeutics, believes the microbiome could be the key to Parkinson’s disease. The disease results when improperly folded alpha-synuclein (αSyn) protein forms aggregates that damage neurons in the brain. Mazmanian’s research has revealed that certain bacteria that produce amyloid proteins can accelerate αSyn aggregation and propagation to the brain.
“What’s really interesting,” Donabedian notes, “is that we’ve found higher levels of these amyloid-producing bacteria in Parkinson’s patients compared to their spouses and matched controls.” Rather than kill the amyloid-producing bacteria, which could throw the entire microbial ecosystem out of balance, Axial is developing small-molecule drugs that inhibit the bacteria from producing the problematic proteins. “The molecules we’ve developed,” Donabedian explains, “inhibit the aggregation and production of amyloids without killing the bugs.”

In July, Axial received a $440,000 research grant from the Michael J. Fox Foundation to create an in vitro model of the enteric nervous system. The model will allow a more detailed look at which cells are implicated in propagating the amyloids from the gut to the brain. Understanding more about the basic biology will help inform dosages and better predict the effect of the drugs.
Axial has demonstrated preclinical proof of concept in animal models and presented early clinical data showing the safety and tolerability of one of the company’s small-molecule candidates. According to Donabedian, efficacy data are expected later this year, and a second, more potent molecule is in the pipeline. “It’s all lining up as it relates to our mechanism,” Donabedian asserts. “We’re showing improvement.”
Optogenetics lights the way to improving deep brain stimulation
Parkinson’s patients currently take oral medication to relieve dyskinesia, but after years on the drug, the benefits eventually wane. Some patients find relief through deep brain stimulation (DBS), a procedure that implants a pacemaker-like device that sends electrical signals to electrodes placed inside the brain. Although DBS can dramatically reduce symptoms, the surgery to implant the device is technically challenging, and it helps only about 8–10% of Parkinson’s patients.
DBS uses electrical pulses to stimulate neurons in the subthalamic nucleus (STN), an almond-size region deep in the brain. It’s unclear exactly how that stimulation leads to a reduction of symptoms. Optogenetics lets researchers genetically program individual neurons to express a light-activated protein and to activate those neurons with pulses of light. In this way, researchers can observe the effects of stimulating very specific neurons, and no others.
At the Center for Interdisciplinary Research in Biology (CIRB), Collège de France, director of research Laurent Venance, PhD, set out to understand on a cellular level how DBS calms Parkinson’s symptoms. “Our hope,” he relates, “was to identify a more superficial target for which we could have less invasive access.”
Parkinson’s motor dysfunction has been associated with hyperactivity of a type of neuron called pyramidal cells, and DBS can settle them down again. “Pyramidal cells of the motor cortex are much more superficial than the STN,” Venance points out, “so it was a good target, and we started there.”
The researchers showed that DBS not only normalized the activity of pyramidal cells in the motor cortex, but also activated neurons called somatostatin-expressing GABAergic interneurons. Using optogenetics, the researchers selectively stimulated these neurons in a mouse model of Parkinson’s disease. The treatment improved the animal’s motor function considerably, suggesting that stimulating these somatostatin-expressing interneurons could be a less invasive target for relieving Parkinson’s dyskinesia.
“You have a unique opportunity with optogenetics to be highly specific in terms of a neuronal subpopulation,” stresses Venance. “You can remotely control a subpopulation of neurons and fine-tune the firing rate. Since it is the firing rate that is at the center of this Parkinsonian state, it was a good tool to specifically manipulate this subpopulation and choose the precise frequency of activation.”
Pooling tumor antigens to supercharge the immune system
Glioblastoma is the most commonly diagnosed brain tumor, and there is no cure. Targeted therapies have proved unsuccessful because the tumors are highly heterogeneous and genetically unstable.
“If you could make a vaccine from the patient’s own tumor, plus tumors of other patients that would have other antigenic profiles, you would have the entire tumor landscape covered,” says Joe Elliot, the managing director of ERC-USA, the American subsidiary of the Epitopoietic Research Corporation (ERC), which is headquartered in Belgium.
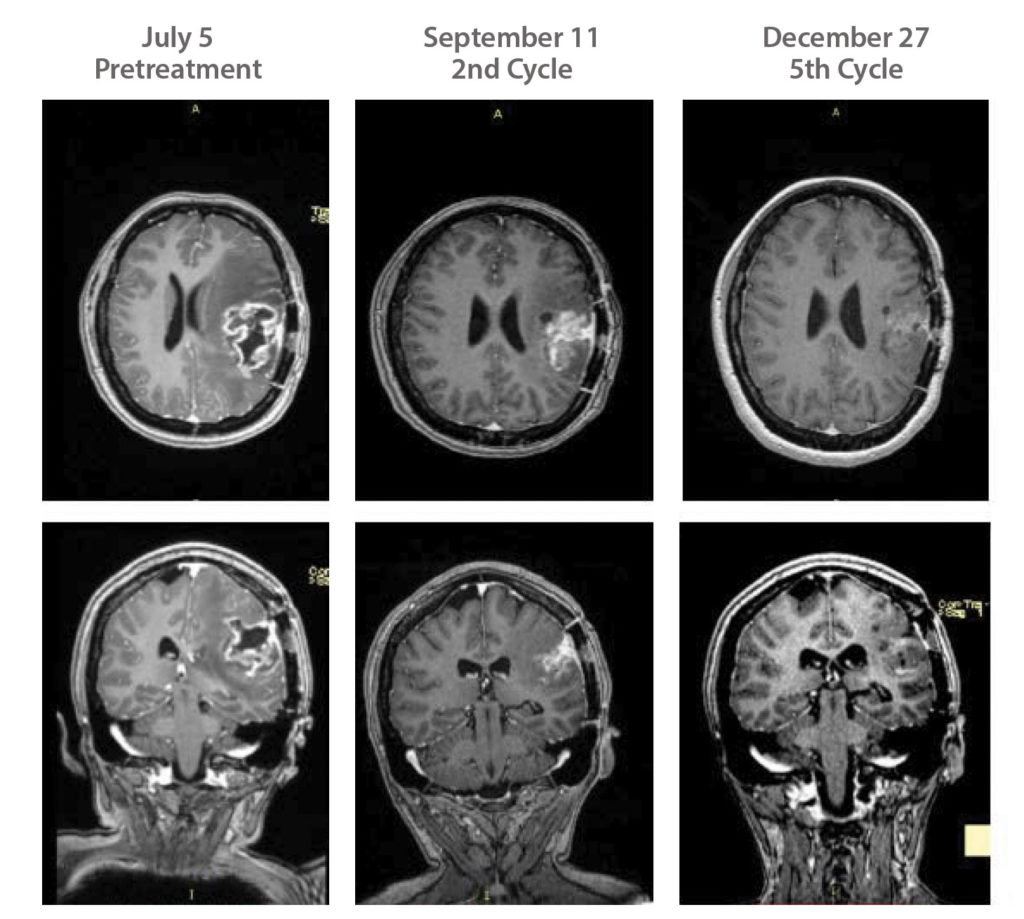
While developing this immunotherapeutic approach, ERC launched a Phase II trial in 2014 at the University of California, Irvine, for its cancer vaccine candidate, ERC-1671. This year, the company added a second site at Dana-Farber Cancer Institute. Located in Boston, Dana-Farber is the largest brain cancer referral center in the United States. To date, 84 patients have been treated, and with the second site, ERC-USA is, according to Elliott, “very optimistic that [it] will be able to significantly accelerate recruitment and complete this Phase II study within 12–18 months.”
ERC-1671 has also been approved for use under the FDA’s Right to Try Act, which allows its use by patients who have already tried all approved treatment options but who don’t qualify for a clinical trial.
Avastin (bevicuzimab) is currently approved to treat relapsed glioblastoma, and average survival is around four months, says Daniela Bota, MD, associate professor of neurology at UC Irvine and the principal investigator of the clinical trial. Patients get randomized to either avastin plus the vaccine or avastin plus placebo. “At the time of recurrence, patients who are getting placebo are allowed to cross over,” Bota details. “We need more work to confirm this, but our patients who have crossed over after progressing on Avastin live around 12 months.”
The personalized treatment contains tissue from the patient’s own tumor as well as tumors from three other patients. The allogeneic tumor tissue is carefully chosen for its major histocompatibility complex mismatch, that is, its potential to generate a strong rejection response. Irradiation prevents cancer cells from proliferating and forming new tumors. “They are still metabolizing, and they are still functioning cells,” explains Elliott. “But the DNA is destroyed, so they can’t reproduce.”
According to Bota, the side effects appear mild. “The patients feel well,” she declares. “They are very excited to participate in this treatment, and the number of patients who live one year or longer is going up.”
Untangling the mechanisms of multiple sclerosis
As powerful as the immune system is for fighting disease, it’s a scourge when turned on the wrong target. In multiple sclerosis (MS), immune cells attack the myelin sheath protecting the nerve cells, disrupting communication between the brain and the body. Symptoms range from muscle weakness and tremors to loss of vision, speech difficulties, and unsteady gait.
Recently, a type of immune cell called T helper 17 (Th17) emerged as a possible driver of autoimmune diseases, including MS. In mouse models of MS, researchers successfully staved off disease symptoms by inhibiting Th17 cell differentiation.
Th17 cells produce the cytokine IL-17, and drugs targeting IL-17 are currently used to treat certain autoimmune conditions, including psoriasis and some types of arthritis. However, clinical trials testing the anti-IL-17 drug secukinimab in autoimmune uveitis, an eye disease related to MS, failed to show efficacy.

Uveitis is being researched in mouse models by Rachel Caspi, PhD, head of the Immuoregulation Section and chief of the Laboratory of Immunology at the National Eye Institute. Working with mice that are prone to the disease, she was surprised to discover that knocking out the gene for IL-17A did not affect their disease symptoms.
“That started raising question marks,” Caspi says. “We hypothesized that in mice that are susceptible, there might be a circuit operating which compensates for lack of IL-17.” Caspi and colleagues thought that this circuit might, like many biological mechanisms, be part of a system that provides redundancy to protect against harmful loss of function. “Cytokines are not there to give us autoimmune disease, they are there to protect us against pathogens,” she explains. “In that context, compensation by other cytokines makes sense.”
The team found that IL-17A dampened the pathogenic activity of Th17 cells through a feedback system. IL-17A signals the cells to produce more IL-24, which slows down the release of other inflammatory cytokines by the Th17 cells.
Caspi says that at this point, it is a “leap of faith” that the findings in mice could translate to improved treatments for MS. In human cells, IL-17A exerts the same feedback control over Th17 cells’ release of cytokines, but that finding hasn’t been tested in patient samples yet. But if inhibiting IL-17 in humans does cause Th17 cells to pump out more cytokines, that might suggest better ways to silence the harmful inflammation wrought by the cells.

